
Major salt concentrate production methods
Seaweed burning method (Moshio-yaki)
Details of the method are not clear and so there are various ideas
- ash salt was made by hardening the ash of burnt seaweed using seawater
- salt concentrate was produced by pouring seawater onto the ash salt
- salt concentrate produced by pouring seawater onto layers of seaweed was boiled down
The generally accepted view is that seaweed was used to produce salt concentrate (seaweed covered by seawater was dried under the sun, and the salt that crystallized on the surface of the seaweed was dissolved by pouring on seawater).
Banked salt-terrace method (Agehamashiki-enden)
Banked salt-terraces were located above sea level. The terraces were made by flattening the ground, hardening the base with clay and then applying a layer of sand.
Seawater carried manually was sprayed over the sand.
The sun and the wind evaporated the water, leaving salt-encrusted sand which was scooped up and put into a tank to concentrate the salt (the concentrator). Seawater was poured into the concentrator to produce a dense salt concentrate.
This method could not be used in bad weather or during winter, so the operation period was spring through autumn. Salt is still produced using this method in Noto Peninsula in Ishikawa Prefecture.

Work procedures at a banked salt-terrace
Seawater was carried and sprayed onto the sand on the terrace.
After the sand dried, it was scooped up and put into the concentrator.
Seawater was poured into the concentrator to dissolve the salt crystallized on the sand.
Salt concentrate drained from the bottom of the concentrator. [This was boiled down to crystallize the salt.]
The sand was taken out of the concentrator and spread on the field again.
Channeled salt-terrace method (Irihamashiki-enden)
One major difference between the channeled salt-terrace method and the banked salt-terrace method was that seawater was not carried manually but poured into the field by exploiting the tidal differences in the sea level.
Another difference was that the sand was wetted with seawater using capillary action. For the channeled salt-terrace method, a dyke was constructed on tidal flats which were shallow for a great distance, and terraces were made at a height midway between low tide and high tide.
In the channeled salt-terrace method, seawater flowed into the channels and spread around the top of the sand by capillary action. The sun and the wind evaporated the water, leaving salt-encrusted sand which was scooped up and put into a tank to concentrate the salt (the concentrator). Seawater was poured onto the sand in the concentrator to produce salt concentrate.
This epoch-making method exploiting the tidal differences in the sea level was developed on the coast of the Setonaikai Sea in the mid-seventeenth century and remained in use until the 1960s.

Work procedures at a channeled salt-terrace
Sand was spread onto the terrace.
Seawater was sprayed onto the sand to enhance capillary action.
The sand was churned to aid evaporation.
After the sand dried, it was scooped up and put into the concentrator.
Seawater was poured into the concentrator to dissolve the salt crystallized on the sand.
Salt concentrate drained from the bottom of the concentrator. [This was boiled down to crystallize the salt.]
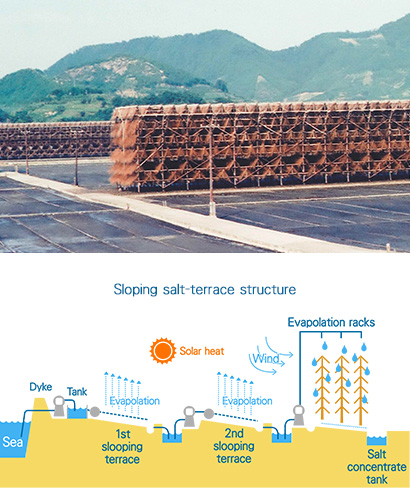
Sloping salt-terrace method (Ryukashiki-enden)
The sloping salt-terrace method replaced the channeled salt-terrace method and was used from the late 1960s to about 1970.
This method used sloping terraces, covered with clay or a layer of plastic sheeting and then by a layer of fine gravel, and evaporation racks made of fine bamboo branches laid out in steps. Seawater was pumped up and flowed down over the first sloping terrace to a second sloping terrace and onto the evaporation racks where the sun and the wind evaporated the water. Salt concentrate was produced by repeating this cycle.
The evaporation racks improved wind evaporation of water because the seawater coated the bamboo branches in a thin film and cascaded down along the layers of branches. The evaporation racks allowed salt to be produced all year round and the sloping salt-terrace method obviated the need to carry heavy sand unlike the channeled salt-terrace method. The only requirement was a flow of seawater, so this method dramatically cut back on labor.
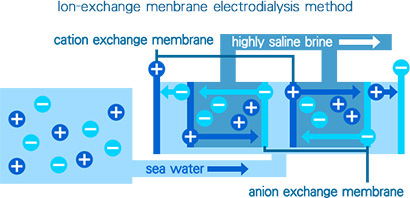
Ion-exchange membrane electrodialysis method
The ion-exchange membrane electrodialysis method produces salt concentrate from seawater using electrical power. This method is based on the principle that the salt in seawater exists as separated electrically-charged positive ions such as sodium, magnesium and calcium ions and electrically-charged negative ions such as chloride ions.
The salt concentration in seawater is about 3.5%. The ion-exchange membrane electrodialysis method is able to produce salt concentrate with a concentration of between about 15% and 20%. This concentrate is boiled down to crystallize the salt.
Other countries in addition to Japan that use the ion-exchange membrane electrodialysis method for salt production are Korea and Taiwan.
The ion-exchanger has two electrodes at the two ends. Placed between these electrodes are a series of alternating cation and anion exchange membranes. The cation exchange membranes allow only positive ions to pass through and the anion exchange membranes permit only negative ions to pass through.
When a direct electric current flows between the two electrodes through the seawater-filled tank, positive sodium, magnesium and calcium ions migrate to the negative electrode, and similarly, but in reverse, negative ions, such as chloride ions, migrate to the positive electrode.
Migrating positive ions such as sodium ions are stopped by the anion exchange membranes, and negative ions such as chloride ions are repelled by the cation exchange membranes. As a result, highly saline brine (with a salt concentration of 15% to 20%) and low saline brine (with a salt concentration of approximately 2%) are trapped between alternating pairs of the membranes.
The salt concentrate is transferred to a evaporation system and the low saline brine is returned to the sea.
Studies on the ion-exchange membrane electrodialysis method began around 1950. Compared with salt production using salt terraces, the ion-exchange membrane electrodialysis method realized remarkably higher productivity of both land and labor and is independent of the weather.
Ion-exchange membranes
The ion-exchange membranes are between 0.1mm and 0.2mm thick and have minute perforations (with a diameter of between 1 and 2 millionths of a millimeter). These membranes have useful properties of allowing the passage of ions such as Na+、Cl-、Ca2+ and Mg2+.
Ion-exchange membranes are widely used in the production of pharmaceuticals and foodstuffs, for example, to produce medical-use water (for injections), to produce drinking water from brine, to remove salt from baby's milk concentrate and soy sauce, and to remove acidity from fruit juice.
